how does a car battery work chemistry
This is the chemistry used in a typical car battery. How does a car battery work learn from the basics where we use and battery and how batteries work.

How A Car Battery Works Basic Working Principle Youtube
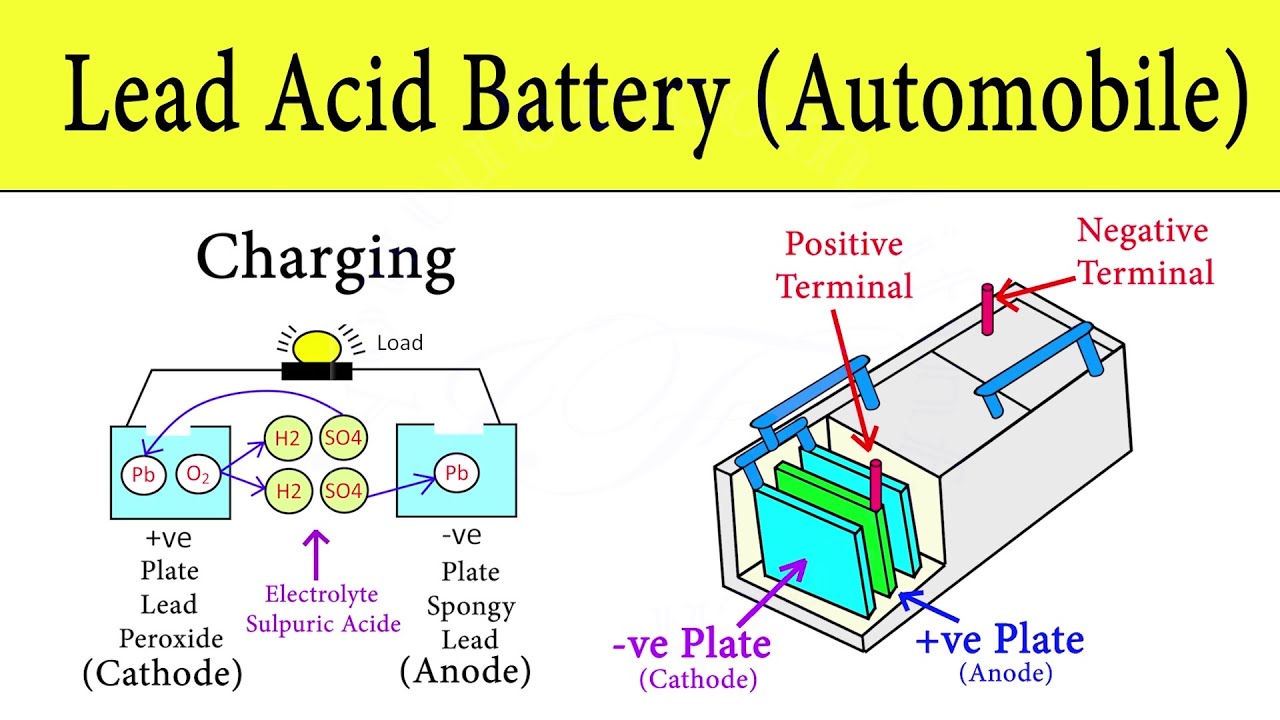
When the battery is in use lead oxide in the cathode reacts with sulphuric acid to form lead sulfate PbSO4.

. Lead-acid battery rechargeable. The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. When the battery is in use lead oxide in the cathode reacts with sulphuric acid to form lead sulfate PbSO4.
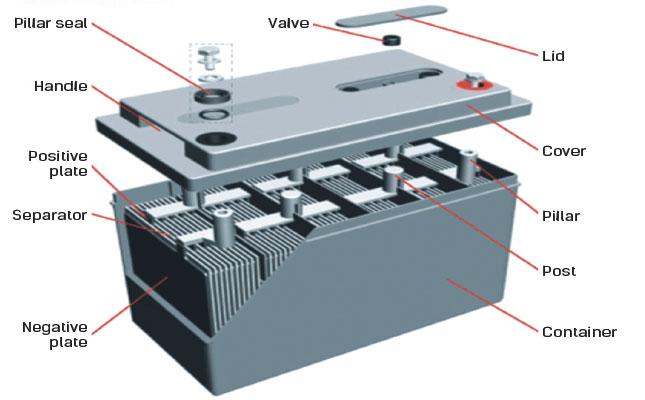
The chemical components of a car battery also known as a lead-acid battery are lead dioxide PbO2 in the cathode lead Pb in the anode and the solution is sulphuric acid H2SO4. A 12-volt automotive battery contains six cells connected in series. In this electro-chemical process four materials react with each other.
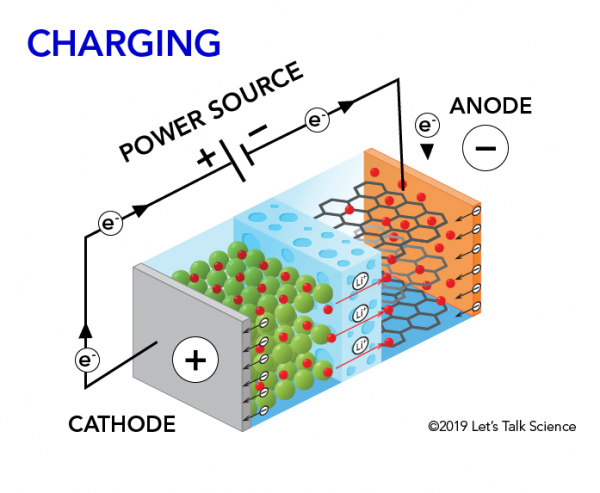
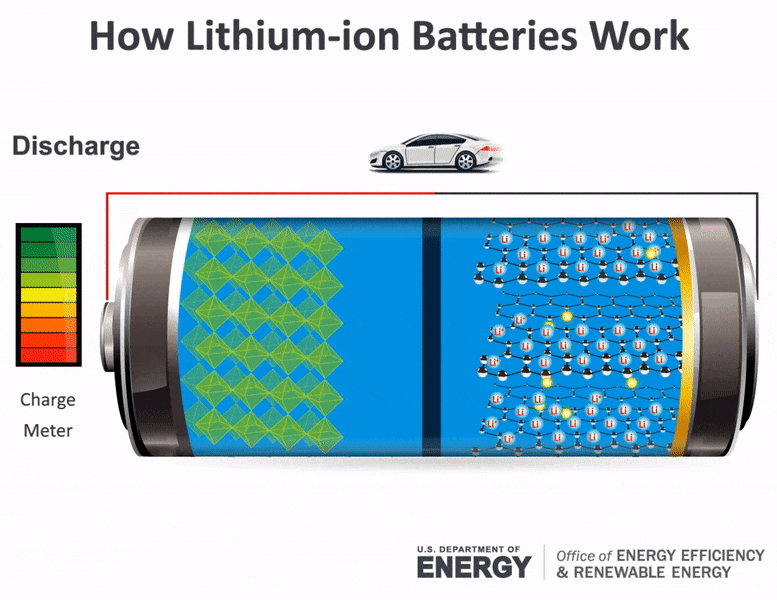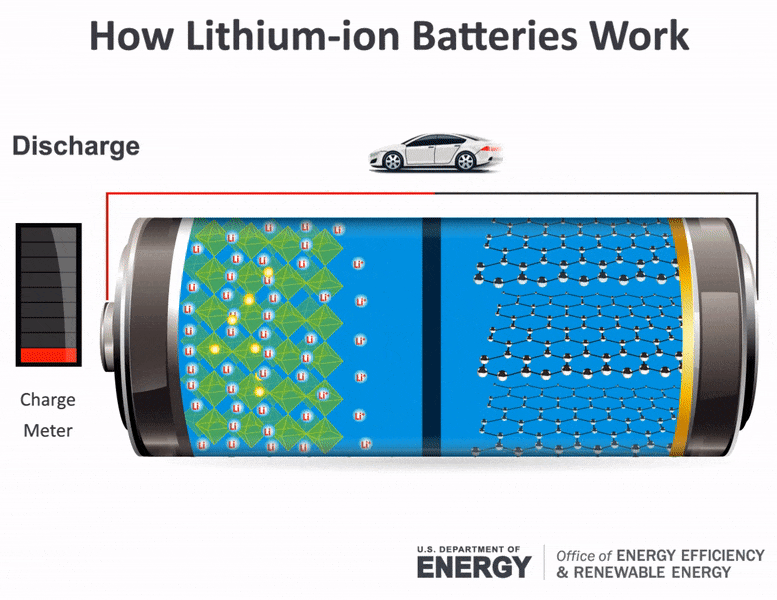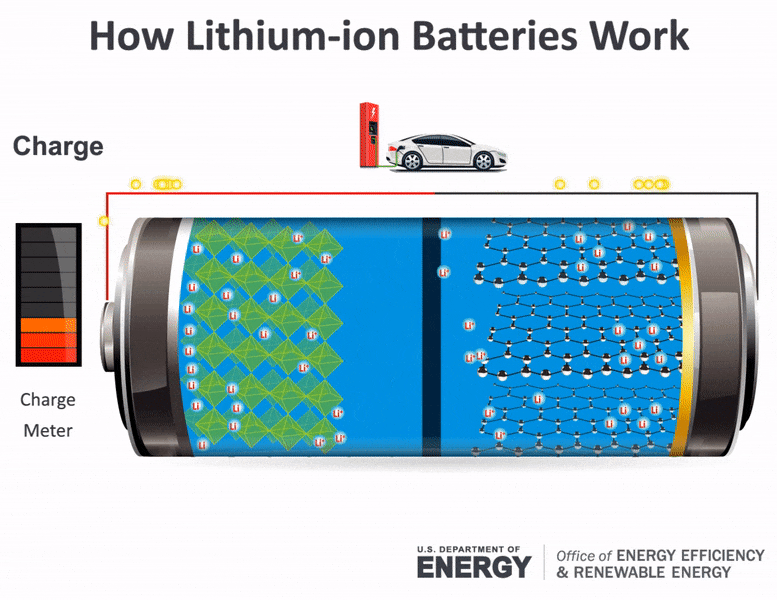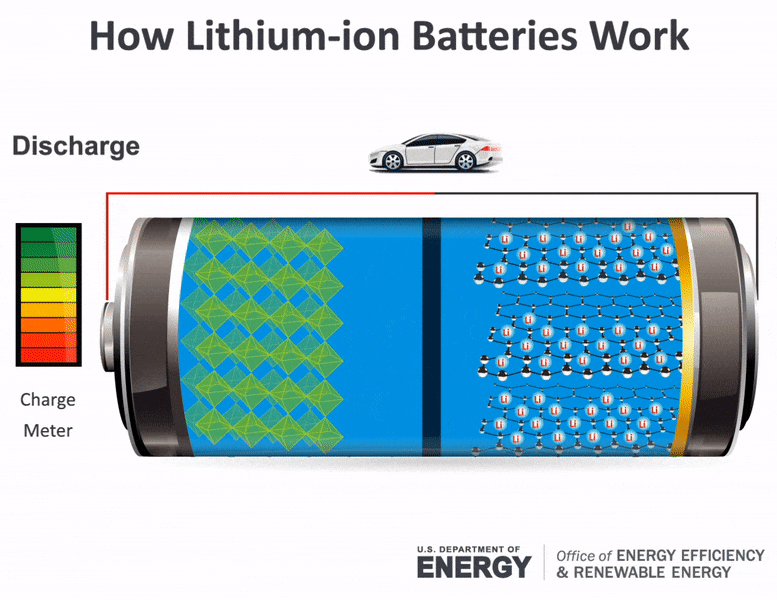
With thanks to Squarespace for sponsoring this video. The movement of the lithium ions creates free electrons in the anode which creates. How does a car battery work chemistry.
The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. How does a car battery work chemistry. The anode and cathode store the lithium.
Hydrogen H Oxygen O 2 Lead Pb Sulfur S Connection of an external consumer starts the chemical reaction in the battery. Inside the battery are 3 important things. The electrodes are usually made of lead dioxide and metallic lead while the electrolyte is a sulfuric acid solution.
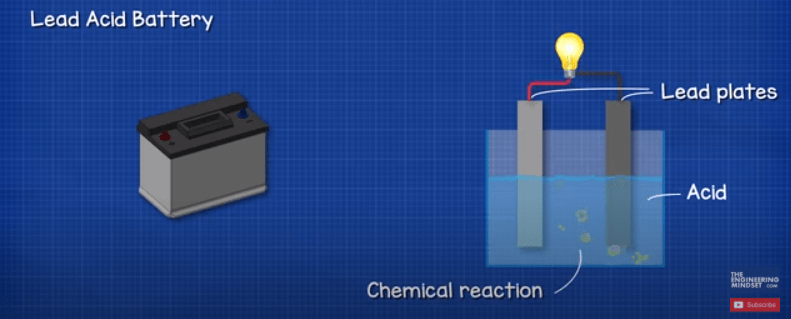
A car battery uses lead-acid technology to turn chemical energy into electricity. These are called the. A car battery stores energy in chemical form and converts it into electrical energy.
An anode electrolyte and a cathode. The chemical energy results from the lead-acid reaction that begins the whole process. So the cathode will be connected to the positive terminal of the battery while the anode will be connected to the negative terminal of the battery.
This causes the voltages of each battery to add. The general way that a battery works is that when an electronic circuit is connected to the battery electrons are allowed to flow. Most standard car batteries contain six cells that are situated in a row inside the plastic casing.
It accomplishes this through the usage of cells which contain and store the energy until needed. The chemical components of a car battery also known as a lead-acid battery are lead dioxide PbO2 in the cathode lead Pb in the anode and the solution is sulphuric acid H2SO4. After every six months check the specific gravity of electrolyte fluid to test the car battery for dead cells.
The Chemistry of How A Car Battery Works The chemical solution Sulfuric acid given as H2SO4 which is a compound of hydrogen sulfur and oxygen The cathode lead dioxide given by the chemical annotation PbO2 a compound of lead and oxygen The anode Lead which is represented by the chemical. The electrolyte a mixture of sulfuric acid H 2 SO 4 and. The best way to understand these reactions is to see them for yourself.
And the electrolyte which separates these terminals. How does a car battery work chemistry. There are 2 connectors that go out of the battery.
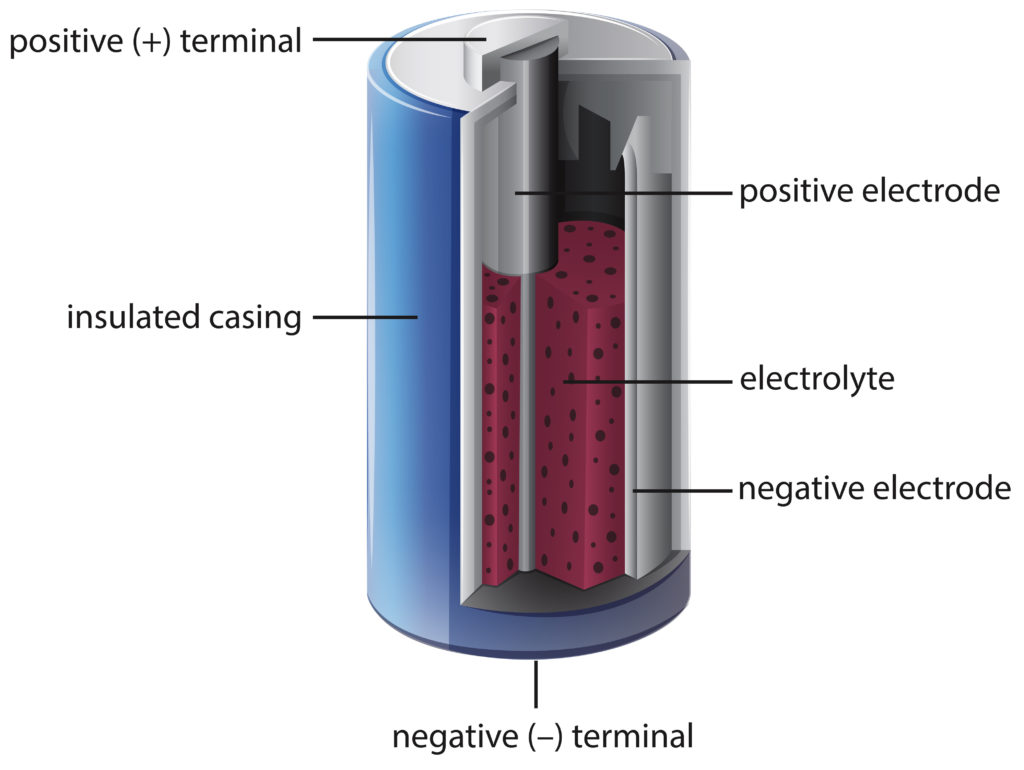
Every battery is made up of three parts. Two terminals made of different chemicals typically metals the anode and the cathode. A battery is a device that transforms chemical energy to electric energy and that is the basic way a car battery works.
The car battery contains six separate cells and every cell has 21 V power. The chemical reaction in the battery will automatically run when the anode and cathode are connected. It accomplishes this through the usage of cells which contain and store the energy until needed.
How does a car battery work learn from the basics where we use and battery and how batteries work. With thanks to Squarespace for sponsoring this video. The lithium ions move back from the cathode to the anode.
There are three main components of a battery. A car battery is actually 6 smaller batteries that are lined up in series. A car battery uses lead-acid technology to turn chemical energy into electricity.
Once any car battery cell is over the battery needs replacement. As such the car battery is categorised as a. Battery chem is an american invention that is considered to be a green technology.
If any of these cells are dead In such a case the battery cant work correctly. A battery is made up of an anode cathode separator electrolyte and two current collectors positive and negative.
What Kind Of Acid Is In A Battery This Is How Your Car Works

How Much Does A Replacement Car Battery Cost
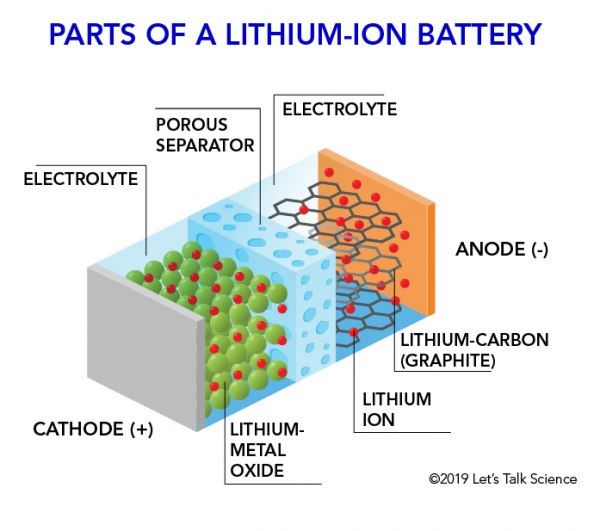
How Does A Lithium Ion Battery Work Let S Talk Science

Battery Working Principle How Does A Battery Work Electrical4u

How Do Batteries Work A Galco Tv Tech Tip Youtube
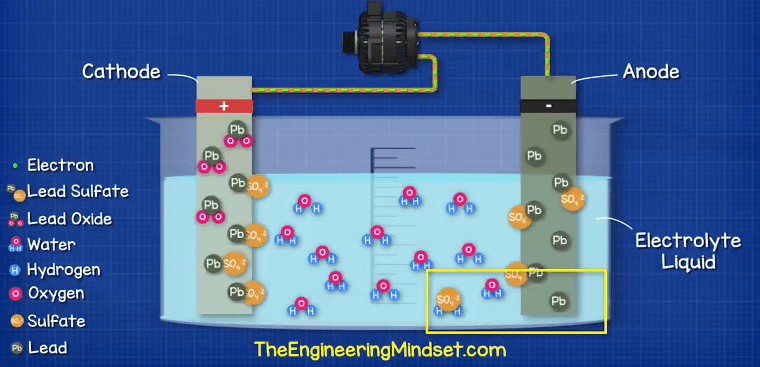
How A Car Battery Works The Engineering Mindset
Science Made Simple What Are Batteries And How Do They Work
How A Car Battery Works The Engineering Mindset
Lead Acid Battery How Car Battery Works Automobile Battery Working Principle Animation Youtube

Working Of Lead Acid Battery Lead Acid Secondary Storage Battery Electrical4u
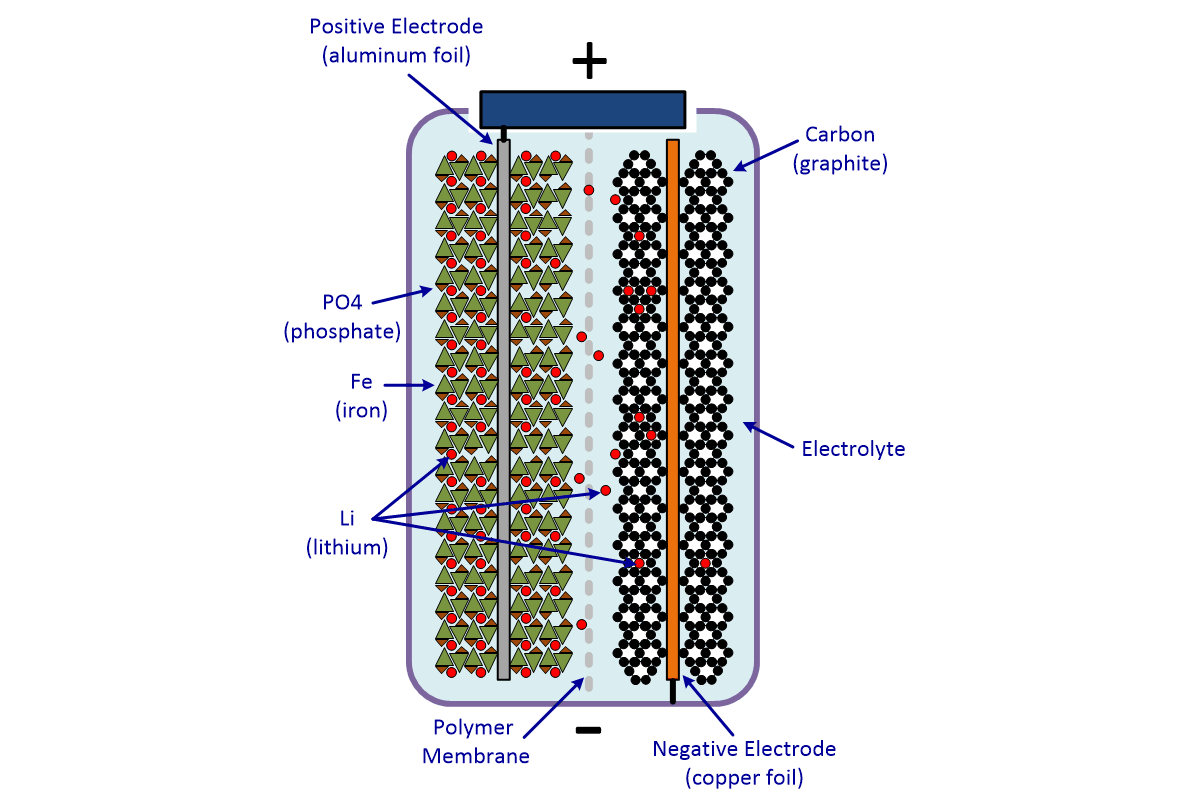
Electric Car Battery Chemistry What S The Difference Carexpert

How A Lithium Ion Battery Works Inverted Energy
How Does A Car Battery Work Mach 1 Services

Battery 101 How Does A Car Battery Work Firestone Complete Auto Care

Working Of Car Batteries Askiitians Blog One Place For All Updates On Iit Jee Medical Exams